We have seen Borax decoration all over the internet and wanted to try them, but with Borax banned in the UK, we had to use a borax substitute to make our pipe cleaner cystal decorations. These are super easy to make and the kids can manipulate the pipe cleaners all by themselves.


This post contains affiliate links. If you make any purchases after clicking one of these links, I will make a small commission (hopefully enough to keep me in tea all year) – at no extra cost to you! This allows me to keep creating and sharing free tutorials and content for you. Thank you!
Materials
- Pipe cleaners
- Borax alternative
- Water
- Food colouring
How to Make Pipe Cleaner Crystal Decorations

Start by shaping some decorations out of the pipe cleaners. We did snowflakes, hearts, trees and spirals (wrapped around pencils).
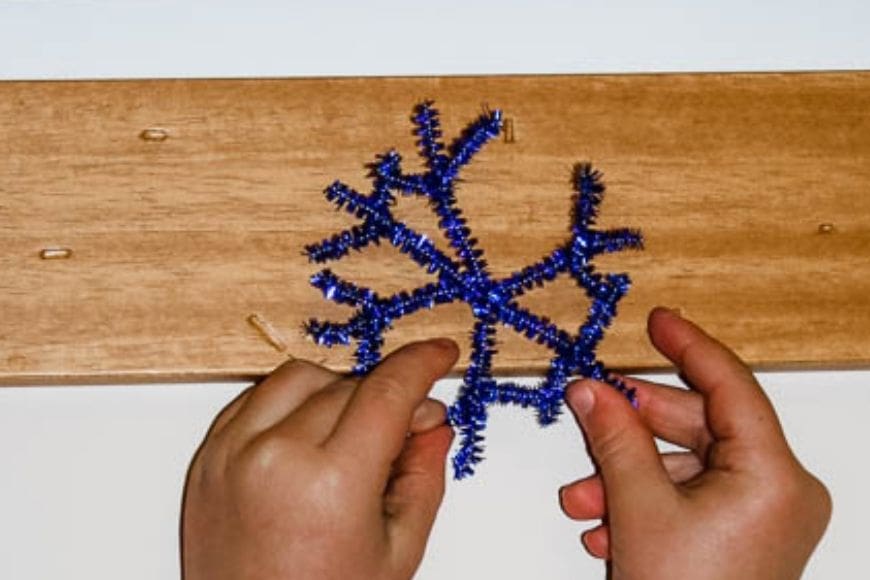

Then mix your borax solution with boiling water and add food colouring of your choice. I used about 6 tablespoons to approximately one pint, but I just poured it in (I am not an exact type of person).


The Science Part
Crystals are solids that are formed by a regular, repeating pattern of molecules connected together. Most crystals come in geometric shapes with sharp, straight edges and smooth sides.
Crystals can form when a supersaturated liquid that contains a dissolved mineral cools. In this activity, a supersaturated solution was made using hot water and a borax alternative (a soft crystal). The hot water caused the water molecules to move further away from each other so that more of the borax could dissolve into the solution. Once the solution reaches a point where it cannot dissolve any more borax, it becomes supersaturated. As the solution cools, the water molecules come closer together again causing the forming borax crystals to cling to the pipe cleaner.








Comments are closed.